“From Water to Oxygen: Could Life Thrive Beyond Earth?”

Can We Make Oxygen from Water—Elsewhere in the Universe?
On Earth, we often say forests are the “lungs of our planet,” but the real oxygen powerhouses are microscopic: phytoplankton in the oceans produce over 50% of our atmospheric oxygen through photosynthesis. The process is simple—water (H₂O) and carbon dioxide (CO₂) are converted into oxygen (O₂) using sunlight.
But what about other worlds? If we discover water on another planet or moon, could oxygen be produced there too—naturally or through human intervention?
Oxygen from Water: How Does It Happen?
1. Photolysis: Splitting Water with Light
Ultraviolet (UV) radiation from stars can break apart water molecules in an atmosphere—a process called photolysis. This releases hydrogen (which often escapes into space), leaving behind oxygen. Over time, this can cause an abiotic (non-life-related) build-up of O₂ and ozone (O₃) in a planet’s atmosphere arXiv.
- Pros: No life is required; oxygen could accumulate naturally.
- Cons: Without a constant source (like plant life or oceans), the oxygen may react with other elements or escape back into space.
2. Known Worlds with Water or Water Potential
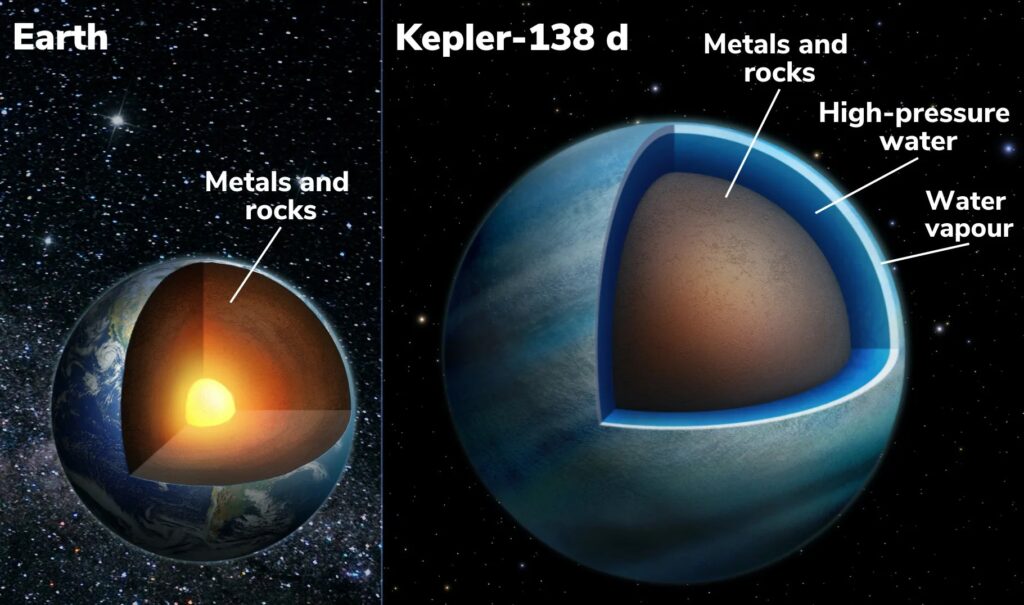
K2-18b
– A “super-Earth” about 2.6 times Earth’s radius located ~110 light-years away.
– Confirmed water vapor and clouds in the atmosphere thanks to Hubble and James Webb Telescopes NASA ScienceNASA.
– Surface conditions unknown, but it’s likely warm and water-rich—possibly a candidate for photolytic oxygen production.
GJ 9827 d
– A sub-Neptune (~2 Earth radii) with detected water vapor via Hubble transit observations arXiv.
– Its atmosphere may contain hydrogen and water—potential ingredients for abiotic oxygen.
LHS 1140 b
– A temperate super-Earth (~5.6 Earth masses) that might host an ocean and a nitrogen-rich atmosphere The Sun.
– With sufficient water and UV light, oxygen could accumulate over time.

3. Ice-Covered Worlds and Subsurface Oceans
NASA has identified 17 exoplanets that might hold subsurface oceans beneath ice shells—like Jupiter’s moon Europa Space. These environments could allow water interaction with rock—possibly leading to oxygen and hydrogen production through chemical reactions (like radiolysis). However, such oxygen tends to get trapped beneath the ice.
4. Can We Detect Oxygen Remotely?
Detecting oxygen or ozone in exoplanet atmospheres is challenging:
- O₂ and O₃ as biosignatures: Their presence can suggest life—but abiotic photolysis can also generate these Phys.orgarXiv.
- We need to look at oxygen alongside other gases like methane to distinguish a biological signature.
- Upcoming telescopes (ELT, JWST, NASA’s LIFE mission) aim to identify these biosignature mixtures arXivPNAS.
5. Could We Engineer It?
In principle, if a world has abundant water and sunlight:
- Humans could install UV-powered photolysis systems to split water and create oxygen.
- However, scale matters: our atmosphere holds roughly 10¹⁸ kilograms of O₂. Achieving that volume artificially would require massive infrastructure and vast water reserves.
Why This Matters
- Understanding habitability: Even abiotic oxygen, if misinterpreted, could be mistaken for life unless carefully analyzed.
- Preparing for exploration: If humans one day settle on Mars or icy moons, oxygen generation from local water could support terraforming or life support.
- Scientific curiosity: Exoplanets like K2-18b and LHS 1140 b offer laboratories for studying how water and oxygen interact on other worlds.
Summary Table
| World | Water Evidence | O₂ Potential | Notes |
|---|---|---|---|
| K2-18b | Water vapor, clouds | Moderate | JWST signs of CH₄, CO₂, DMS |
| GJ 9827 d | Atmospheric water | Possible | Smallest planet with H₂O |
| LHS 1140 b | Ocean candidate | Good | Temperate, nitrogen-rich |
| Ice-covered exo-woods | Subsurface oceans | Low | Oxygen trapped, not free air |
Final Thoughts
Yes—oxygen can form from water, both naturally through photolysis and potentially through engineered means. Planets with water vapor or oceans—like K2-18b, GJ 9827 d, and LHS 1140 b—offer strong cases to study this process. But oxygen alone isn’t proof of life; we need contextual clues—other gases, atmospheric composition, and environmental conditions.
Our next step? To use powerful telescopes and space missions to sample these atmospheres, distinguishing between worlds with life and those with chemistry alone.


Thinking about checking out the VIP side of things at afunvip. Anyone have any experience with it? Seen some tempting bonuses… afunvip
Naseebetgame… Gave it a quick browse. The layout is decent, and looks like some potentially fun stuff to do. Give it a quick look and see: naseebetgame
789plink…sounds mysterious! Hope it’s a lucky link to wins! Need quick withdrawals, yeah? 789plink
For the lives I’m playing, I look for 58jllive it’s safe, secure and helpful. Its site is super easy to access!
16betcom… yeah, I know them. Been using their site for a while now. Pretty reliable. Go check them out: 16betcom
On the go? MV9Bet’ll probably work on mobile. Time to check it out. mv9bet
пылесос дайсон беспроводной спб [url=https://pylesos-dn-6.ru/]pylesos-dn-6.ru[/url] .
пылесос dyson купить в спб [url=https://pylesos-dn-7.ru/]pylesos-dn-7.ru[/url] .
где купить дайсон в санкт петербурге [url=https://dn-pylesos-3.ru/]dn-pylesos-3.ru[/url] .
dyson пылесос купить [url=https://dn-pylesos-3.ru/]dn-pylesos-3.ru[/url] .
dyson gen5 купить в спб [url=https://pylesos-dn-6.ru/]pylesos-dn-6.ru[/url] .
ремонт пылесоса дайсон в спб [url=https://pylesos-dn-7.ru/]ремонт пылесоса дайсон в спб[/url] .
дайсон где купить в спб [url=https://pylesos-dn-7.ru/]pylesos-dn-7.ru[/url] .
официальный магазин дайсон в санкт петербурге [url=https://pylesos-dn-6.ru/]pylesos-dn-6.ru[/url] .
дайсон официальный сайт спб [url=https://pylesos-dn-7.ru/]дайсон официальный сайт спб[/url] .
дайсон пылесос купить спб [url=https://pylesos-dn-6.ru/]pylesos-dn-6.ru[/url] .
дайсон официальный сайт [url=https://pylesos-dn-7.ru/]дайсон официальный сайт[/url] .
пылесос дайсон беспроводной купить в спб вертикальный [url=https://pylesos-dn-6.ru/]pylesos-dn-6.ru[/url] .
дайсон купить спб [url=https://pylesos-dn-kupit-8.ru/]дайсон купить спб[/url] .
dyson оригинал спб [url=https://pylesos-dn-kupit-10.ru/]pylesos-dn-kupit-10.ru[/url] .
вертикальный пылесос дайсон купить спб [url=https://pylesos-dn-kupit-8.ru/]pylesos-dn-kupit-8.ru[/url] .
dyson пылесос [url=https://pylesos-dn-kupit-10.ru/]dyson пылесос[/url] .
dyson пылесос беспроводной купить спб [url=https://pylesos-dn-kupit-8.ru/]pylesos-dn-kupit-8.ru[/url] .
санкт петербург магазин дайсон [url=https://pylesos-dn-kupit-10.ru/]санкт петербург магазин дайсон[/url] .
купить дайсон в санкт петербурге [url=https://pylesos-dn-kupit-8.ru/]pylesos-dn-kupit-8.ru[/url] .
пылесосы дайсон спб [url=https://pylesos-dn-kupit-10.ru/]пылесосы дайсон спб[/url] .
dyson спб официальный [url=https://pylesos-dn-kupit-8.ru/]pylesos-dn-kupit-8.ru[/url] .
dyson магазин в спб [url=https://pylesos-dn-kupit-10.ru/]dyson магазин в спб[/url] .
1xbet giri? 2025 [url=https://1xbet-giris-23.com/]1xbet giri? 2025[/url] .
t.me/s/top_onlajn_kazino_rossii [url=https://t.me/s/top_onlajn_kazino_rossii/]t.me/s/top_onlajn_kazino_rossii[/url] .
1xbet giri? g?ncel [url=https://1xbet-mobil-1.com/]1xbet-mobil-1.com[/url] .
1xbet giri? yapam?yorum [url=https://1xbet-mobil-5.com/]1xbet giri? yapam?yorum[/url] .
1xbet ?yelik [url=https://1xbet-yeni-giris-2.com/]1xbet-yeni-giris-2.com[/url] .
авто новости [url=https://avtonovosti-1.ru/]avtonovosti-1.ru[/url] .
1xbet g?ncel giri? [url=https://1xbet-mobil-1.com/]1xbet-mobil-1.com[/url] .
t.me/s/top_onlajn_kazino_rossii [url=https://t.me/s/top_onlajn_kazino_rossii/]t.me/s/top_onlajn_kazino_rossii[/url] .
1xbetgiri? [url=https://1xbet-mobil-5.com/]1xbetgiri?[/url] .
1xbet g?ncel [url=https://1xbet-giris-23.com/]1xbet g?ncel[/url] .
автоновости [url=https://avtonovosti-1.ru/]автоновости[/url] .
1xbet giri? adresi [url=https://1xbet-yeni-giris-2.com/]1xbet giri? adresi[/url] .
1xbet giri? adresi [url=https://1xbet-mobil-1.com/]1xbet-mobil-1.com[/url] .
1xbet tr giri? [url=https://1xbet-mobil-5.com/]1xbet-mobil-5.com[/url] .
t.me/s/top_onlajn_kazino_rossii [url=https://t.me/s/top_onlajn_kazino_rossii/]t.me/s/top_onlajn_kazino_rossii[/url] .
1x giri? [url=https://1xbet-giris-23.com/]1xbet-giris-23.com[/url] .
журналы автомобильные [url=https://avtonovosti-1.ru/]avtonovosti-1.ru[/url] .
one x bet [url=https://1xbet-yeni-giris-2.com/]1xbet-yeni-giris-2.com[/url] .
1xbet mobil giri? [url=https://1xbet-yeni-giris-2.com/]1xbet-yeni-giris-2.com[/url] .
1xbet resmi sitesi [url=https://1xbet-yeni-giris-2.com/]1xbet-yeni-giris-2.com[/url] .
авто новости [url=https://avto-zhurnal-4.ru/]avto-zhurnal-4.ru[/url] .
журналы для автолюбителей [url=https://avto-zhurnal-4.ru/]avto-zhurnal-4.ru[/url] .
журнал про авто [url=https://avto-zhurnal-4.ru/]журнал про авто[/url] .
автоновости [url=https://avto-zhurnal-4.ru/]автоновости[/url] .
авто новости [url=https://avto-zhurnal-4.ru/]avto-zhurnal-4.ru[/url] .
карниз с приводом для штор [url=https://karnizy-s-elektroprivodom77.ru/]karnizy-s-elektroprivodom77.ru[/url] .
электрокарниз [url=https://karnizy-s-elektroprivodom77.ru/]karnizy-s-elektroprivodom77.ru[/url] .
электрокарнизы москва [url=https://karnizy-s-elektroprivodom77.ru/]электрокарнизы москва[/url] .
электрические гардины [url=https://karnizy-s-elektroprivodom77.ru/]karnizy-s-elektroprivodom77.ru[/url] .
автоматический карниз для штор [url=https://karnizy-s-elektroprivodom77.ru/]автоматический карниз для штор[/url] .
automaty online [url=https://casino-cz-1.com/]casino-cz-1.com[/url] .
casino bonus za registraci [url=https://casino-cz-1.com/]casino-cz-1.com[/url] .